Van Der Waals Equation a and B Units
B has units of Lmol. P a V 2 V b R T.
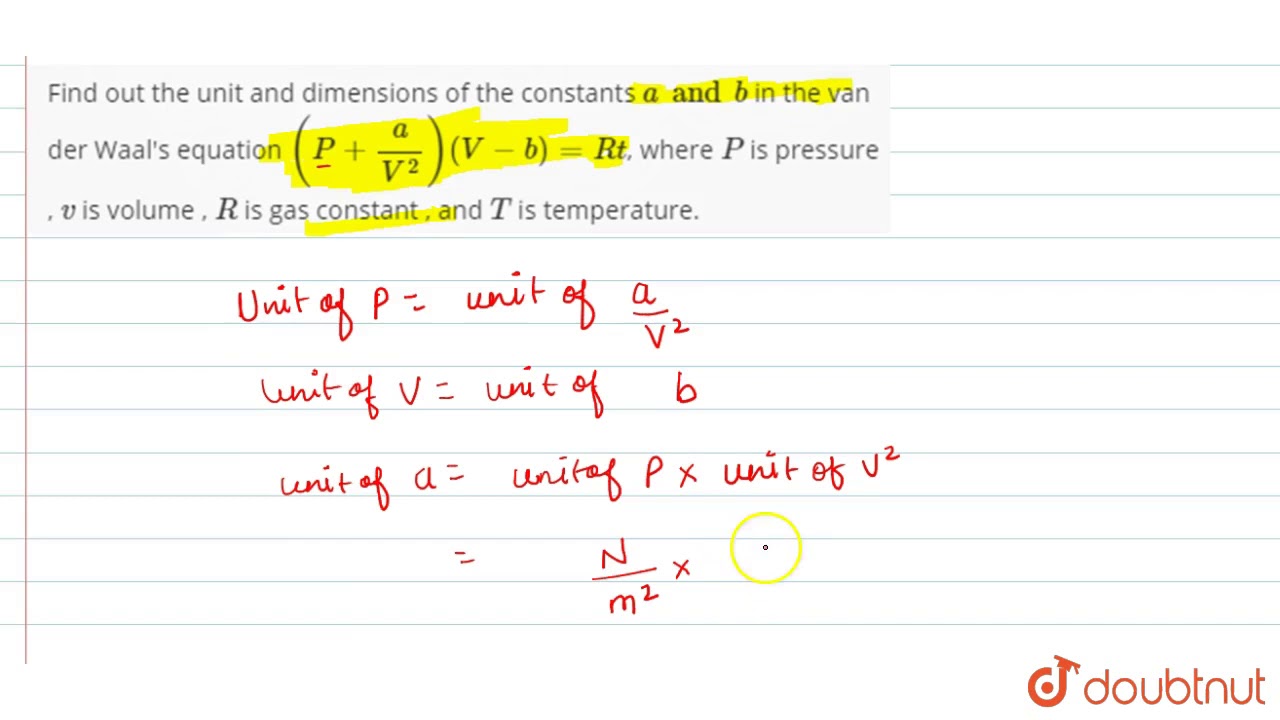
Find Out The Unit And Dimensions Of The Constants A And B In The Van Der Waal S Equation P Youtube
B determines the volume occupied by the gas molecules which depends upon size of molecule.

. Greater the value ofa more will be the attractive forces. The van der Waals equation is an equation of state that corrects for two properties of real gases. The expression for Van der waals equation is given as following.
The van der Waals constants a and b are characteristic of the substance and are independent of temperature. 227 rows It is usually referred to as the van der Waals equation of state. The factor - nb accounts for the volume occupied by the gas molecules.
The constant a is the measure of the magnitude of intermolecular attractive forces between the particles. Applying principle of homogeneity Unit of P Unit of a V² Unit of a unit of P unit of V² Nm² m³² N m4 Unit of V unit of b m³ Hence unit of a Nm4 And unit of b m³ Van der Waals equation is P aV² V-b RT Here P is pressure and V is volume. A is related to the magnitude of the attractive forces among the molecules of a particular gas.
A has units of. Van der Waals constant b. Together the variable aV 2 represents the correction factor for the pressure due to the attractive forces.
B 006380 dm3 mol-1 answer831. Unit of b litre mol¹. The excluded volume of gas particles and attractive forces between gas molecules.
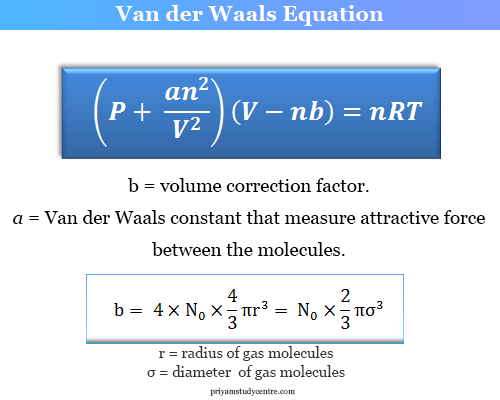
Where the variable b is a constant and relates to the actual volume of the gas molecules themselves and a is a constant and relates to the strength of the attractive forces between gas molecules. Van der Waals equation derivation is based on correcting the pressure and volume of the ideal gases given by the Kinetic Theory of Gases. Helium is a monatomic gas and each mole of helium contains 6022 10 23 atoms the Avogadro constant N A.
Giaiotti and Fulvio Stel 1 Regional Meteorological Observatory via Oberdan 18A I-33040 Visco UD - ITALY Abstract This lecture deals with a more general form of the equation of state called van der Waals equation which gives a better description of reality both under the conceptual and. Hereof What is B in real gas equation. Van der Waals consTanTs for gases The van der Waals equation of state for a real gas is Pn22aVVnbnRT where P is the pressure V the volume T the temperature n the amount of substance in moles and R the gas constant.
Unit of a and is atm lit² mol². Van der Waals equation showing correction for attraction and volume. The constants a and b represent the magnitude of intermolecular attraction and.
P a n 2 V 2 V n b n R T. Van der Waals equation is an equation relating the relationship between the pressure volume temperature and amount of real gases. Van der Waals Equation Derivation.
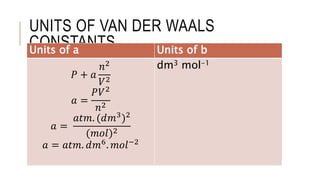
For a real gas containing n moles the equation is written as. Compressibility factor Van der Waals Equation. Units of Van der Waals Constants.
If a gas behaves ideally both a and b are zero and van der Waals equations approaches the ideal gas law PVnRT. P dfracan2V2 Rightarrow a dfracp times V2n2. According to n mole of gas van der Waals equation is given as.
The constants a and b are called van der Waals constants. The van der Waals equation is frequently presented as. P correction V 2an 2 Unit of aatm litre 2mol 2V correction nbUnit of blitre mol 1Unit of ba litre mol 1atm litre 2mol 2 atm litre mol 1.
P dfracan2V2V - nb nRT Where a and b are the van der Waals constant. A 5562 bar dm6 mol-2. B has units of Lmol.
For a real gas containing n moles the equation is written as. The van der Waals equation is an equation of state that corrects for two properties of real gases. Two-dimensional 2D materials 12 and the associated van der Waals vdW heterostructures 34567 have provided great flexibility for integrating distinct atomic layers beyond the traditional.
Van der Waals equation is an equation relating the relationship between the pressure volume temperature and amount of real gases. B is generally much smaller in magnitude than a. P an2 V 2V nb nRT P a n 2 V 2 V n b n R T.
Constant b adjusts for the volume occupied. According to the Tanners General Chemistry webpage The van der Waals Equation if you are considering 1 mole of a substance then the formula is PaV2V-b RT For n moles Pan2V2V-nb nRT The Science HQ website Van der waals equation the constants a and b represent pressure and volume corrections respectively. The values of a and b generally.
20 b The quantity has units of pressure and is called the internal pressure Tey. Van der Waals constant a. The van der Waals constant for C2H6 are.
It is a measure of the strength of attractive forces in substance which are small for gases and large for liquids. 172 x 10-3 Question PHYSICAL CHEMISTRYCalculate the pressure exerted by 10 mol C2H6 at 1000 K in 100 cm3 using a the ideal gas. It is also called co-volume or excluded volume.
From the above equation we can observe that. Since b corresponds to the total volume per mole occupied by gas molecules it closely corresponds to the volume per mole of the liquid state whose molecules are closely layered. The Van der Waals constant b volume can be used to calculate the Van der Waals volume of an atom or molecule with experimental data derived from measurements on gases.
Unit of a can be calculated as. Unit ofa L 2 mol-2. Where P is the pressure of the real gas V is the volume of the real gas n is the number of moles of real gas R is Universal gas constant a and b are Van der waals constant.
They have positive values and are characteristic of the individual gas. The excluded volume of gas particles and attractive forces between gas molecules. The constant a gives the idea of the magnitude of attractive forces between the molecules of the gas and b is the measure of effective volume occupied by the gas molecules in the van der Waals equation.
Pan2V2 VnbnRT P. Units of Van der Waals equation Constants. The van der Waals equation is frequently presented as.
Significance of Van der Waals constants. For helium b 237 cm 3 mol. The van der Waals equation of state by Dario B.
Another derivation is also used that is based on the potentials of the particles. The constant a provides a correction for the intermolecular forces. The equation of state for one mole of a van der Waals gas is RT p 02 a 0-6 a Briefly comment on the physical significance of a and b.
Physical Significance of a and b. Pressure defect for n moles of a gas is given as. P anV 2 Vn - b RT.

The Units Of The Van Der Waals Constant A Are Youtube
Comments
Post a Comment